Researchers Offer a New Approach to Lower Fossil Fuel Use
The increasing levels of atmospheric CO2 have caused a number of harmful effects on the climate system of our planet.
An excessive amount of anthropogenic CO2 has been released into our atmosphere due to the heavy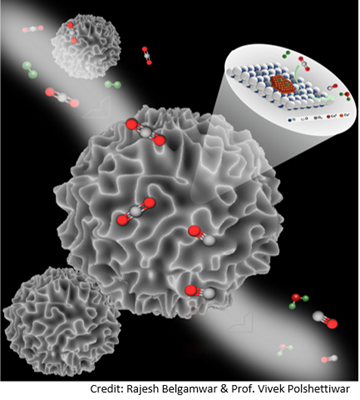 usage of fossil fuels in industrial processes and other human endeavours. A group of Mumbai-based Tata Institute of Fundamental Research (TIFR) scientists have suggested employing hydrogen-aided redox pathways to reduce CO2 in such operations.
usage of fossil fuels in industrial processes and other human endeavours. A group of Mumbai-based Tata Institute of Fundamental Research (TIFR) scientists have suggested employing hydrogen-aided redox pathways to reduce CO2 in such operations.
This highly high atmospheric CO2 concentration has suffered severe effects on our planet’s climate system. However, CO2 may be a valuable carbon source when making valuable compounds and fuels.
Noble metal catalysts have been the subject of numerous reports, but their use has been constrained by both their poor catalytic activity and expensive price. Cu-based catalysts are among the most adaptable in the non-noble metal catalyst family and have good potential in many industrial processes. The research team notes that copper’s low Tammann temperature and the ensuing surface migration cause nanoparticles to sinter during the reaction, restricting their activity and long-term stability.
The team presented a catalyst for CO2 to CO conversion with active copper sites loaded on titanium oxide-coated dendritic fibrous nano-silica (DFNS/TiO2-Cu), using the Strong Metal Support Interactions (SMSI) and defect site cooperativity concepts.
“DFNS/TiO2’s fibrous shape and high surface area enabled for improved dispersion and high Cu NPs active site loading. It outperformed all copper-based thermal catalysts regarding catalytic CO2 reduction with CO productivity. According to researchers, the copper nanoparticles were retained securely anchored on the surface of the support by the defect-controlled strong metal-support interactions between Cu and TiO2, which also provided good catalytic stability.
“The DFNS/TiO2-Cu demonstrated excellent catalytic performance, and in-situ mechanistic analyses suggested the possibility of defects in regulating the metal-support solid contacts. Researchers believe this strategy could result in the design of catalytic systems with various active locations and flawed supports.
The team’s experiments showed that copper sites and Ti3+ sites interacted strongly, resulting in good stability and dispersion of the active copper sites.
The researchers have published an article in the American Chemical Society Journal ACS Publications based on their study. The team comprises Vivek Polshettiwar, Rajesh Belgamwar, Rishi Verma, Tisita Das, Sudip Chakraborty, etc. (India Science Wire)
