Containing the Deadly COVID-19: Viability of Pooling Technologies
With the outbreak of COVID-19 in early December 2019, top pharmaceutical companies are engaged in a race to come up with the vaccine at record time. However, pharma companies are also sceptical about discovering an effective vaccine on time, even though scientists worldwide are moving very fast.
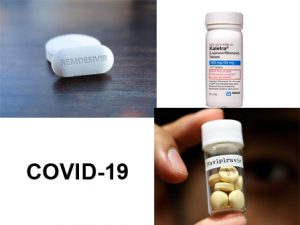
Global pandemic COVID-19 is now in 199 countries with reported confirmed cases above two million and mounting death tolls in thousands every day. In the absence of vaccines and medicines to prevent or treat COVID-19, many options have been proposed including repurposing older medicines. For instance, it is reported that in India, we have successfully treated a patient using a combination of HIV, swine flu and malaria drugs. China and Japan had used ant-influenza drug favipiravir and obtained encouraging outcomes. However, the drug remdesivir, developed to treat Ebola virus disease (EVD), is now considered as the best available option to treat COVID-19.
At the same time, there are reports coming in questioning the efficacy of these treatments. For instance, a study conducted among 199 COVID-19 patients in China shows that the treatment with lopinavir–ritonavir drug (kaletra) combination led to no different outcomes when compared to standard care in terms of time taken for clinical improvement. The study showed that 94 patients who were given lopinavir–ritonavir experienced more adverse gastrointestinal problems, such as vomiting and diarrhoea, as compared to those who received standard care, which included supplemental oxygen, ventilator support and antibiotics. Likewise, there is scepticism about the effectiveness of favipiravir in patients with severe illness. Hence, all the currently used drugs are working on a ‘trial and error’ method and detailed trials have to be done before zeroing in on a specific drug for treatment.
Also Read : Gilead To Donate More Than Million Doses Of Its Experimental Anti Coronavirus Drug
Vaccines are always a very profitable segment for the pharmaceutical industries. The global sale in vaccines alone totalled US$ 54 billion in 2019 and is currently predicted to be near US$ 60 billion in 2020.
With the outbreak of this novel virus in early December 2019, top pharmaceutical companies are seeing it as a gold mine and are engaged in a race to come up with the vaccine at record time. A survey by Genetic Engineering & Biotechnology News (GEN) has revealed that 35 active drug development programmes are currently on in North America, Europe and China, which include pharmaceutical giants like GlaxoSmithKline, Merck, Johnson & Johnson, Eli Lilly, Pfizer and Sanofi, and small start ups like Moderna Inc., Regeneron, etc.
There are also reports coming in that even pharmaceutical companies are sceptical about discovering an effective vaccine on time even though scientists worldwide are moving very fast. Various reasons are being adduced for this. One important reason is that the commercial markets for vaccines and antiviral treatments are weak. The potential patient pool for clinical trials dries up once a new infectious outbreak subsides, so investment can be frozen in partly completed projects. Moreover, the pool of medical customers for a drug can be short-lived, if a seasonal epidemic quickly disappears, offering little chance to recoup an investment of what could be hundreds of millions of dollars. According to available reports Merck & Co. is the only major drug company that still has a vaccine for a novel pathogen, the Ebola immunization Ervebo isn’t working on a vaccine for coronavirus because of the huge complexities involved in ensuring a success.
The obsession of mainstream pharmaceutical industries with patents generates a concern that whether the R&D outcome would be affordable to the masses is doubtful and cannot be guaranteed. Civil society groups are already alarming governments to ensure access to these innovations. The remarks of Mikael Dolsten, Chief Scientific Officer and President, Worldwide Research, Development and Medical of Pfizer Inc., is worthwhile to note: “We have to come to terms that one company, one vaccine, one test or one medicine will not be an effective solution to overcoming the tremendous task at hand. This pursuit requires a crucial multi-pronged approach with a deep collaboration and partnership across the health innovation ecosystem – from the academic community, industry partners, policymakers and regulatory bodies. A challenge like coronavirus requires every organization to bring forward its resources and expertise to manage health risks that evolve daily”. Dolsten called the entire industry to leverage their collective expertise and capabilities. This shows the realization even among pharma corporation that winner takes all approach R&D model cannot meet the need of the hour, i.e., a fast and effective product development.
Against this back ground, the proposal by Costa Rica for the creation of a global pooling mechanism for rights in the data, knowledge and technologies useful in the prevention, detection and treatment of the coronavirus/COVID-19 pandemic acquires relevance. It calls for voluntary assignments of technologies with the existing and future rights of patent, designs, regulatory test data, knowhow, cell lines, copyrights and blueprints for manufacturing diagnostic tests, devices, drugs, or vaccines.
Moreover, technology should be provided either for free access or licensing on reasonable and affordable terms in every member country.
Also Read : Covid 19 In India Immense Role For Healthcare Field Workers
Patent pooling, though initiated in digital technologies, has been extended to public health also to improve access. Unitaid established the Medicines Patent Pool (MPP) in 2010 as the first public health patent pool. This was for antiretroviral (ARV) treatment which enabled generic manufacturers in low-income countries to produce drugs before the respective patent expiry. The pool was later expanded to treatments for tuberculosis and hepatitis C, and is being expanded from time to time. Pooling patent has a crucial role in ensuring access to drugs. For instance, take the experience of HIV treatments. The pharmaceutical companies with patents were able to bar lower-priced generic versions from becoming available. It took global campaigning and international action for this situation to change. As a result, companies have licensed the patents for all HIV regimens recommended by WHO to the MPP, which enables qualified generic producers to produce low-cost medicines. Today, MPP licenses allow generic companies to provide ARVs to around 90 per cent of the people living with HIV in low- and middle-income countries. Prices for first-line treatment have dropped to US$ 75 per patient per year. The number of people receiving treatment was only 611 000 globally in 2000, whereas it has been extended to 21.7 million people in 2017.
However, the pool, as proposed currently, is different from all the pre-existing models and cannot be even considered as a ‘patent pool’ seeing the expansive list. First of all, it is not referring to a single technology, but all the technologies related to coronavirus, and not only the existing ones but also future ones too. Further, it is not limiting to patents only. It requires regulatory test data, know-how, cell lines, then to even copyrights and blueprints for manufacturing diagnostic tests, devices, drugs, or vaccines. All these make the implementation of the mechanism more complex. Moreover, the proposal is not making any clear distinction between the technology required for innovation and manufacturing. Many countries require technologies for the production of various existing medical products to combat COVID-19.
Open Drug Discovery (ODD) could be another option to be tried out. The initiative aims to provide affordable healthcare to the developing world by providing a global platform for drug discovery bringing scientists, researchers, doctors, students and hospitals to work for a common cause. It is being lauded as the best approach for pharmaceutical industry in cases where, if acting alone, would not reap the expected results. As rightly pointed out by Pfizer’s Mikael Dolsten, first we understand that one company, one vaccine, one test or one medicine is not possible to contain this deadly virus. This is the time to try out different models apart from the existing traditional ones to face this extraordinary situation.
